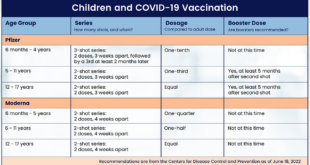
FDA Approves At-Home Flu Vaccine: Convenience and Accessibility – A game-changer for flu prevention, the FDA’s approval of at-home flu vaccines marks a significant shift in how individuals can protect themselves against the seasonal virus. This move promises greater accessibility and convenience, potentially boosting vaccination rates and mitigating the impact of flu outbreaks.
The at-home vaccine, available in both nasal spray and injection forms, allows individuals to administer the vaccine themselves, eliminating the need for a doctor’s visit or a trip to the pharmacy. This convenience is expected to be particularly appealing to busy individuals, families with young children, and those who may have difficulty accessing traditional vaccination services.
FDA Approval and Significance
The FDA’s approval of an at-home flu vaccine marks a significant milestone in the fight against seasonal influenza. This development promises to revolutionize how individuals access and receive their flu vaccinations, potentially increasing vaccination rates and mitigating the impact of flu outbreaks.
Factors Considered by the FDA
The FDA’s decision to approve an at-home flu vaccine was based on a rigorous evaluation process, ensuring the vaccine’s safety, efficacy, and ease of administration. The key factors considered by the FDA include:
The vaccine’s safety and efficacy were paramount, ensuring it provides protection against the influenza virus while minimizing adverse effects.
The FDA assessed the ease of administration, considering factors like the delivery mechanism, self-administration instructions, and the potential for user errors.
The FDA also evaluated the stability and storage requirements of the vaccine, ensuring it remains effective and safe during transportation and storage.
The agency reviewed the manufacturing process, ensuring it meets quality control standards and produces consistent batches of the vaccine.
Vaccine Technology and Administration: FDA Approves At-home Flu Vaccine
The FDA’s approval of an at-home flu vaccine marks a significant step in making vaccination more accessible and convenient. This new vaccine is designed for self-administration, offering individuals greater control over their healthcare choices.The at-home flu vaccine is a nasal spray formulation, similar to some existing flu vaccines.
This means it is administered through the nostrils, bypassing the need for injections.
Administration Process
The administration process is straightforward and designed for ease of use. Here’s a breakdown:
- The vaccine comes in a single-use, pre-filled nasal spray applicator.
- Users are instructed to gently insert the applicator into one nostril and spray the vaccine.
- The process is repeated for the other nostril.
- The vaccine is self-administered, with no need for healthcare professionals.
The instructions provided with the vaccine will include detailed information on the proper technique and precautions.
Advantages and Disadvantages
At-home vaccination offers several advantages over traditional methods:
- Convenience:It eliminates the need for appointments and travel to healthcare facilities, making vaccination more accessible for individuals with busy schedules or limited mobility.
- Increased Accessibility:The at-home format can increase vaccination rates by reaching individuals who may not have easy access to healthcare providers, such as those in rural areas or those who are homebound.
- Reduced Costs:The potential for lower administration costs could make vaccination more affordable for some individuals.
However, at-home vaccination also presents some potential disadvantages:
- Potential for Misadministration:There is a risk of improper administration if users do not follow the instructions carefully.
- Limited Supervision:The absence of a healthcare professional could limit the ability to address any potential complications or side effects.
- Storage and Handling:Maintaining the vaccine’s potency requires proper storage and handling, which may pose challenges for some individuals.
Availability and Distribution
The at-home flu vaccine is expected to be widely available, with distribution channels encompassing pharmacies, healthcare providers, and online retailers. However, there may be initial limitations based on manufacturing capacity and regulatory approvals.
Investigate the pros of accepting Nick Chubb NFL Retirement Rumors Explained in your business strategies.
Distribution Channels, FDA approves at-home flu vaccine
The vaccine will be distributed through various channels to ensure widespread accessibility.
- Pharmacies: Major pharmacy chains, such as CVS, Walgreens, and Rite Aid, are expected to be key distribution points for the at-home flu vaccine. Their established infrastructure and customer base make them ideal locations for convenient access.
- Healthcare Providers: Physicians’ offices, clinics, and hospitals will also offer the vaccine, allowing patients to receive it during routine visits or appointments.
- Online Retailers: Online platforms like Amazon and other e-commerce sites may also sell the vaccine, providing an additional option for convenient access.
Access for Underserved Populations
To ensure equitable access, initiatives and programs are being developed to address the needs of underserved populations.
- Community Health Centers: These centers often serve low-income and uninsured individuals, providing crucial access to healthcare services, including vaccinations.
- Mobile Vaccination Clinics: Mobile clinics can reach remote or underserved communities, bringing vaccination services directly to those who might otherwise lack access.
- Government Programs: Government programs like the Vaccines for Children (VFC) program provide free vaccines to eligible children. These programs can be expanded to include the at-home flu vaccine, increasing accessibility for vulnerable populations.
Last Word
The FDA’s approval of at-home flu vaccines represents a major step forward in public health. By making vaccination more accessible and convenient, this innovation has the potential to significantly impact flu season outcomes. While the vaccine’s safety and efficacy have been rigorously tested, it’s crucial for individuals to follow the administration guidelines carefully and consult with healthcare professionals if they have any concerns.
The future of flu prevention may well be in our own hands, with the at-home vaccine offering a new path to protect ourselves and our communities.
Quick FAQs
Is the at-home flu vaccine safe and effective?
The at-home flu vaccine has undergone rigorous clinical trials to ensure its safety and efficacy. The FDA’s approval indicates that the vaccine meets its stringent standards.
Who can receive the at-home flu vaccine?
The at-home flu vaccine is generally available to individuals aged 2 years and older. However, there may be specific age restrictions for certain vaccine formulations.
How long does it take for the at-home flu vaccine to be effective?
It typically takes about two weeks for the at-home flu vaccine to provide full protection.
What are the potential side effects of the at-home flu vaccine?
The most common side effects of the at-home flu vaccine are mild and may include soreness at the injection site, headache, or muscle aches.
 CentralPoint Latest News
CentralPoint Latest News